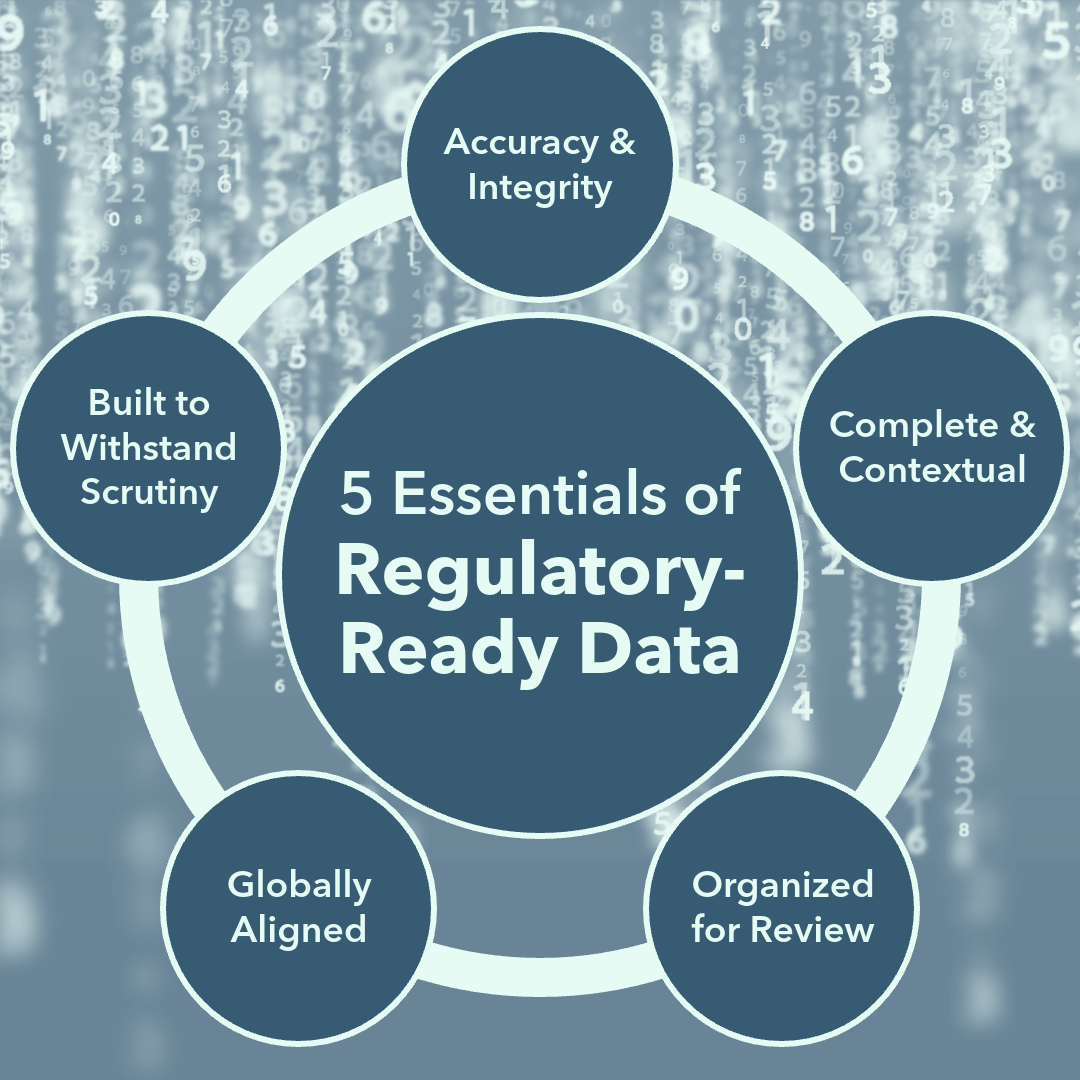
What Does “Regulatory-Ready” Data Look Like?
When it comes to regulatory submissions, not all data is created equal.
Regulators want evidence that is structured, validated, traceable, and globally aligned. Anything less risks delays, deficiency letters, or costly rework. At NMCG, we call this regulatory-ready data.
But what does that mean in practice?
1. Accuracy and Integrity Come First
Regulatory-ready data must be reliable, reproducible, and free of bias or gaps. It should follow Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) standards, backed by documentation that confirms authenticity.
Example: Source data verification ensures trial data matches patient records, and validated third-party labs prevent non-compliant results.
2. Complete and Contextual
Numbers without context raise red flags. Regulatory-ready data includes not only what was measured, but also why and how. Protocols, case report forms (CRFs), and statistical analysis plans must all connect to endpoints that matter.
Key inclusions:
Protocols aligned with study goals
Statistical analysis plans that anticipate reviewer questions
Clear traceability from raw data to final conclusions
Acceptance criteria with scientific and/or clinical rationale
3. Organized for Review
Even the strongest data can stall a submission if it’s poorly presented. Regulatory-ready data is structured, transparent, and easy for regulators to navigate. A clean, logical submission reduces review times and unnecessary follow-ups. NMCG recommends combining summaries and providing detailed data in a format that’s easy to follow.
4. Globally Aligned Standards
Most innovators aim for more than one market. Regulatory-ready data that aligns with both FDA and international requirements (EU MDR, UK regulations, TGA, etc.) eliminates the need to redo full studies for new regions — saving time and money.
5. Built to Withstand Scrutiny
Finally, regulatory-ready data is designed to withstand tough questions. It accounts for risk management, human factors, cybersecurity, and long-term safety considerations—well before regulators or advisory panels raise them.
Why It Matters
Regulatory-ready data is the foundation of efficient approvals/clearances. When submissions are incomplete or disorganized, programs stall. When they’re thorough and strategic, reviews move faster, credibility with regulators increases, and safe, effective products reach patients sooner.
At NMCG, we help clients plan for success from day one—designing trials, monitoring data, and preparing submissions that meet FDA and global expectations. From protocol to final report, our team ensures your data tells a clear, compliant story.