Insights That Move You Forward
Helping you make smarter decisions, avoid costly missteps, & bring safe, effective products to market with confidence
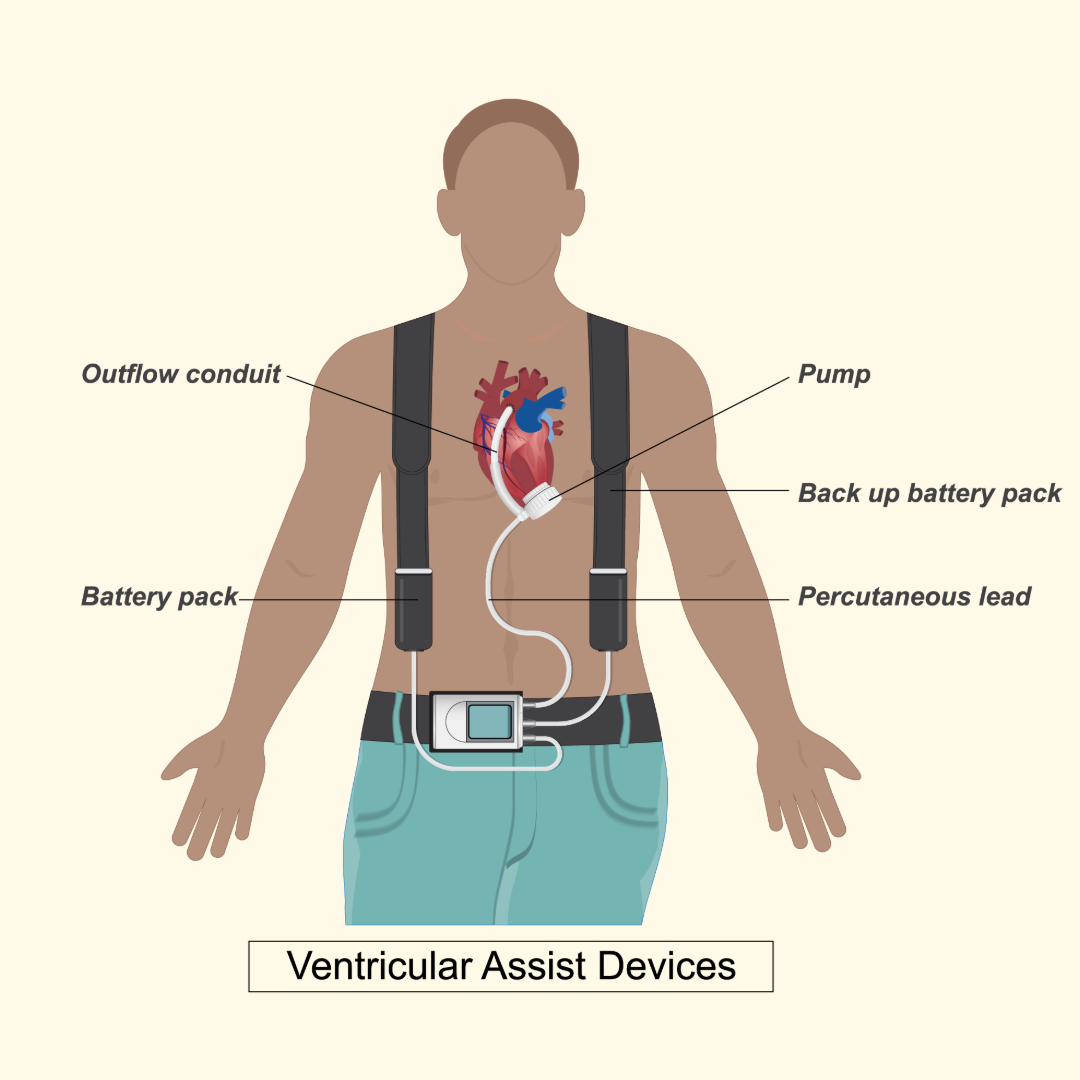
How Combined Mechanical Support Technologies May Improve Cardiac Function: New Research from NMCG’s Kimberly Lamberti, PhD
NMCG’s Kimberly Lamberti, PhD, has published new research in Intensive Care Medicine Experimental examining how ventilator pressure influences mechanical heart pump support in acute heart failure. The study shows that increasing PEEP can improve cardiac and pulmonary vascular function—challenging traditional assumptions and informing care for cardiogenic shock.

The Growing Role of Real-World Evidence in Regulatory Decisions
Real-world evidence (RWE) is reshaping regulatory decisions, supplementing clinical trial data with insights from EHRs, registries, and digital tools. Regulators now rely on RWE to strengthen approvals and post-market surveillance. See how NMCG helps sponsors design data strategies that anticipate this evolving landscape.

Real-World Impact: Poor Data Integrity Examples from Recent Rejections
Regulatory rejections often stem from preventable data integrity failures—like falsified lab results, poor traceability, or incomplete safety documentation. These errors delay patient access and damage credibility. Learn from real-world examples and see how NMCG helps sponsors safeguard submissions from costly pitfalls.

3 Signs Your CRO Strategy Needs Adjustment
Missed timelines, poor data quality, and communication gaps are red flags that your CRO strategy isn’t working. NMCG provides the oversight, project management, and flexibility needed to keep clinical trials compliant, efficient, and on track.

What to Expect from an FDA Pre-Submission Meeting
FDA Pre-Submission meetings offer critical insight into regulatory expectations before you invest time and budget. From strategy planning to meeting facilitation, NMCG helps sponsors maximize feedback, reduce delays, and approach FDA reviewers with confidence and clarity.

Common Protocol Pitfalls and How We Help You Avoid Them
Clinical trial protocols often fail when they’re overly complex, misaligned with regulatory expectations, or impractical for sites and study subjects. These pitfalls cause delays, costly amendments, and wasted resources. NMCG helps sponsors avoid common missteps by designing clear, compliant, and right-sized protocols built for success from the start.

CRO vs. Consulting Firm: What’s the Difference and Why It Matters
Success in clinical development depends on knowing when to engage a CRO and when to rely on a consulting firm. CROs excel at executing trials with precision, while consulting firms guide strategy, submissions, and compliance. Understanding the difference—and when you need both—can mean the difference between costly delays and streamlined market approval.
